
A monkey model of early Alzheimer’s Disease: Moving past complications with rodent models
Almost undoubtedly, you can think of five influential and loved women in your life. With those women in mind, consider that one in every five women and one in every ten American men at the age of 45 are at risk of developing Alzheimer’s disease (AD). As the rates of the disease continue to increase and promising therapies tested in rodents fail in human subjects, it’s clear we need another option. Post-doctoral scholar, Dr. Danielle Beckman in Dr. John Morrison’s laboratory at the California National Primate Research Center (CNPRC) and the rest of their team have developed a monkey model of the earliest phase of AD. By selectively infusing protein fragments linked to Alzheimer’s Disease into the brain of middle-aged female rhesus monkeys, they have induced the earliest phase of AD pathology, the synaptic phase, without neuron death. Morrison’s lab is focusing on middle-aged females, instead of the geriatric populations previously studied, in hopes of identifying a treatment to stop the disease before irreversible degeneration occurs.
Interestingly, some neurons, like those in the prefrontal cortex and hippocampus that are critically important for learning and memory more readily bind Aβ oligomers and are, consequently, more susceptible to these toxic effects than others. In addition, AβOs apparently damage selectively the class of spines that are most plastic and required for learning and memory. In a healthy brain, there is a delicate balance of neuronal anatomy necessary for cellular communication and plasticity underlying learning. In AD, important tools of communication between neurons like synapses and dendritic spines are compromised which leads to cognitive decline and may leave the neuron vulnerable to the next stage of AD- neuron death.
Morrison and Beckman’s team believe that Aβ oligomers are instigating the synaptic damage and by infusing them directly into the brain over a short period of time, they have established a model of AD that isolates the synaptic phase before evidence of pathology indicative of irreversible damage. “That is really powerful”, says Morrison, “we want to be able to isolate the synaptic phase of the disease, because we want to intervene at the synaptic phase and see if we can block its progression.”
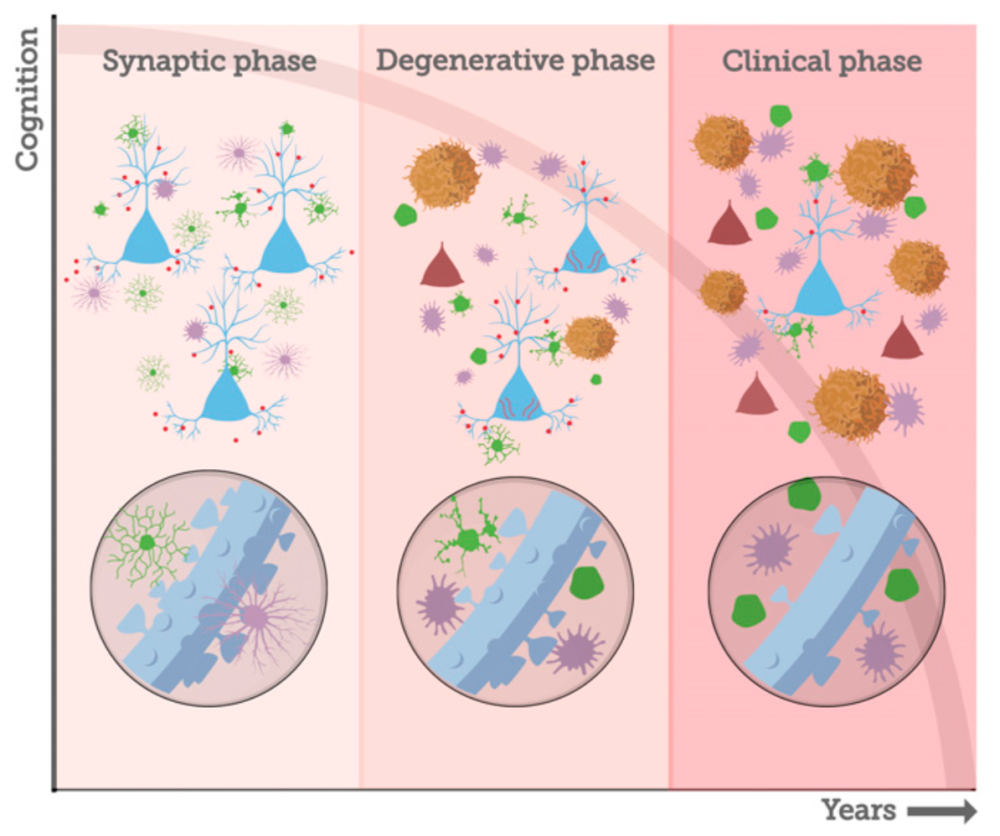
Beckman and Morrison are looking forward to the next steps for their monkey model of AD. There are plans to examine behavioral correlates of synaptic pathology, investigate the known effects of estrogen in AD development, and to test drug therapies capable of stopping AD progression before it reaches the degenerative phase. Primate models of the disease will greatly augment our capacity to connect the dots between testable biomarkers, cognitive decline, and underlying pathology to outline an effective treatment plan for humans in the earliest phase of AD, prior to the extensive irreversible damage that results in dementia.
Corresponding authors:
Dr. Danielle Beckman, California National Primate Research Center, DBeckman@ucdavis.edu
Dr. John Morrison, California National Primate Research Center, JHMorrison@ucdavis.edu
Written by Logan Savidge lesavidge@ucdavis.edu
