ENDOCRINE CORE MOVES TO CENTER FOR HEALTH AND ENVIRONMENT:
As of January 1, 2019, the Endocrine Core headed up by Dr. Bill Lasley and Nancy Gee has moved to the Center for Health and Environment (CHE). They are up and running in their new space and available for business. If you are interested in working with the Endocrine Core you should reach out to Nancy Gee either via email at nagee@ucdavis.edu or by phone at 530.752.2096.
Services

One of the distinctive features of PDL is our ability to work with clients in understanding and interpreting laboratory data as it relates to their NHP colony. Senior staff can provide expertise in experimental design, assay development, interpretation of diagnostic testing, case and colony management.
In addition, we have a strong record of technology transfer having provided training and protocols to numerous visiting scientists who have then successfully implemented assays in their own laboratories.
Some of PDL’s ongoing research interests include the discovery and understanding of new emerging infectious agents, and the application of new, state of the art laboratory diagnostic methodologies. Please contact us to discuss your specific interests.
PDL maintains a large archive of appropriately preserved and stored sera, plasma, DNA, cells and other NHP body fluids and tissues. These materials have been characterized for their reactivity to various agents including SRV, SIV, STLV, SFV, Herpes B, RhCMV, TB, and RRV. We also maintain stocks of virus isolates, cells, proteins, and nucleic acids for similar agents. PDL routinely uses these materials for quality control, process documentation, proficiency, training, and assay optimization and validation.
As sample volume permits, these reagents are available on a recharge basis for sharing with other investigators. Please contact us to discuss your specific interests.
ABSCN-5 Includes: SRV, SIV, STLV, Herpes B Surrogate Marker, Measles
ABSCN-8 Includes: SRV, SIV, STLV, Herpes B Surrogate Marker, Measles, RhCMV, SFV, RRV
ABSCN-Custom Includes: ABSCN-8 and addtional agents by request (SV40, LCV, custom).
The Antibody Screen panels utilize Multiplex Microbead ImmunoAssays to detect the host’s humoral immune response to various infectious agents. The panels simultaneously detect virus-specific antibodies in serum, plasma or other body fluids. The antibody screen assays are run on a Luminex platform using panels of microbeads coated with purified antigens. Each antigen bead has uniquely identifiable spectral signature.
Microbeads are incubated with diluted serum samples so that any specific antibodies present will bind to the matching antigen coated beads. The beads are washed, and reacted with phycoerythrin-conjugated detection reagents. After additional washing, independent gated events for each bead are counted and analyzed in a Luminex cytometer.
The median fluorescence intensity (MFI) of antigen-coated bead sets is compared to control coated beads in each serum. These assays were designed as screening assays with optimal sensitivity and require confirmatory testing by more specific methods.
The minimum sample requirement is 1 ml of serum or plasma sent frozen.

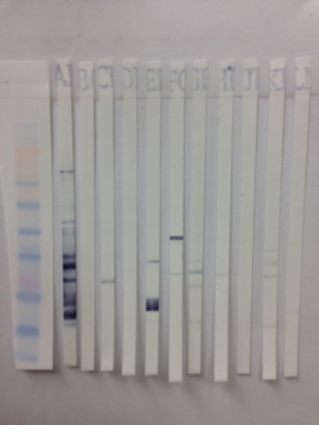
Antibody Screen (ABSCN) results can be confirmed by Western Blot. This method allows visualization of antibody reactivity specific to viral (and non-viral) proteins as separated by molecular weight.
Individual WB’s available for: SRV1, SRV2, SRV5, SIV and STLV. In the WB assay, if specific antibody is present in the serum sample it will bind to the specific antigen bands on the PVDF or Nitrocellulose strip. The presence of antigen-bound antibody is detected by the addition enzyme conjugated detector antibody.
Substrate is added that will yield a color change where the enzyme has been bound (positive for antibody). The minimum sample requirement is 1 ml of serum or plasma sent frozen.
Antibody Screen (ABSCN) results can be confirmed by IFA.
Individual IFA’s available for: Measles, SFV, RRV, SV40, LCV and Varicella.
The Immunofluorescence assay (IFA) can be used to detect antibodies to specific virus in infected cells coated on microscope slides. In this assay, test and control serum samples are reacted on the test antigen infected and uninfected control cells.
If specific antibody is present in the test sera, it will bind to the test antigen infected and not the uninfected control cells. The specific antibody – test antigen binding is detected by binding a secondary, fluorescent conjugated detector antibody. The signal is observed by fluorescence-microscopy.
The minimum sample requirement is 1 ml of serum or plasma sent frozen.
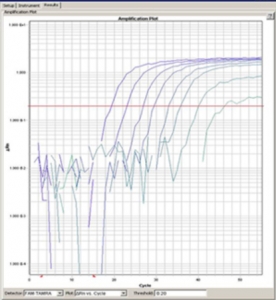
Individual PCR available for: SRV (1-5), SFV, STLV, RRV and SIV.
PCR assays are used to detect viral nucleic acids (DNA, RNA, proviral DNA) in various body fluids, whole blood or tissue. This assay allows us to detect and amplify very small quantities of viral or proviral nucleic acid. The appropriate nucleic acid is extracted from blood or tissue and then amplified in the PCR assay.
All of our current assays use Taqman1 or Real Time PCR. Probes and primers have been designed to amplify specific viral gene sequences.
In addition, probe and primers to amplify the housekeeping gene oncostatin M (OSM) which encodes a cytokine are run in each reaction. The OSM gene is diploid in all primate cells allowing for cell quantification. The OSM signal is used to assure that we obtained amplifiable DNA for all samples.
Although for many viruses a PCR result is diagnostic, for SRV, both PCR and serology are necessary to rule out infection.
The minimum sample requirement is 3 ml of heparinized whole blood sent at room temperature to arrive within 24 hours of collection.
In vitro assays for Interferon Gamma (IFN-g) response to tuberculin antigens represent a refinement of the lymphocyte stimulation assay. As soon as possible following collection, aliquots of heparinized whole blood are stimulated with control and TB antigens in pre-loaded tubes. After 24 hours incubation, the concentration of IFN-g in the supernatant plasma of each aliquot is determined by enzyme immunoassay.
In experimental primate infection models, cell mediated immune responses are detectable in vitro 2 to 4 weeks after infection. IFN-g is a critical cytokine in the cell mediated immune response to tuberculin antigens, including the DTH response measured by the TST, and in the host immune response to infections with tuberculous mycobacteria. Primagam (Prionics, USA Inc, La Vista, NE) is a commercial version of this assay using bovine and avian purified protein derivatives (PPD) as antigens.
Please contact us to arrange to receive instructions and pre-loaded stimulation tubes to be filled, incubated and processed on-site immediately following collection.
Please call for more information about PDL offered ELISA/ EIA’s.
The presence or absence of antibodies in serum or plasma can be determined qualitatively by Enzyme Immuno Assay. In these assays, microtiter plate wells are coated with antigen (typically purified viral lysate). Diluted serum is incubated in the wells. If specific antibody is present it will bind to the antigen. The enzyme conjugated detector antibody is added next. Finally a chromogen reagent is added. The development of color in the wells signifies the presence of specific antibody.
The minimum sample requirement is 1 ml of serum or plasma sent frozen. 
Immunohistochemistry, or IHC, refers to the process of visualizing antigens (e.g. proteins) in cells of a tissue section. Specific antibodies are used to bind specifically to antigens in biological tissues.
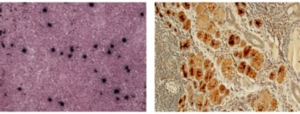
Immunohistochemical staining is widely used in the diagnosis of abnormal cells such as those found in cancerous tumors. Specific molecular markers are characteristic of particular cellular events such as proliferation or cell death (apoptosis). Virus proteins can be visualized in specific tissues and specific cell compartments using this technique. This is particularly useful in cases where suspicion of the infection doesn’t occur until after euthanasia and typical blood and plasma samples are not available.
Immunohistochemistry stains thin slices of formaldehyde-fixed paraffin-embedded (ffpe) tissues on glass slides. Specific antibody (poly- or monoclonal) targeted to the antigen is incubated with the tissue. A secondary antibody, conjugated to an enzyme (e.g. peroxidase) or fluorescent tag is then used to visualize the binding. The enzyme/conjugate catalyzes a reaction that results in colored deposition in the tissue. PDL offers SRV IHC using a monoclonal gp20 antibody that cross-reacts with serotypes 1-5. Please inquire about custom IHC for other pathogens.
In situ Hybridization, or ISH, refers to the process of visualizing specific nucleotide sequences in tissue sections. The tissue is treated to encourage hybridization. A conjugated probe is used to specifically hybridize to the sequence of choice and then visualized. We are currently validating ISH for SRV and other pathogens. This assay is offered on a custom basis.
Other assays are available or can be developed to answer specific research or diagnostic questions. The charge is actual labor plus cost of reagents. Please contact us to discuss.
Some examples include:
- Virus isolation for SIV, SRV, SFV, RhCMV and RRV
- SRV Immunohistochemistry (IHC)
- SRV In-situ Hybridization (ISH)
- PCR typing for SRV 1-5
- Quantitative PCR
- RNA PCR
- Quantitative antibody titers Cytokine /Chemokine Detection and Quantitation Assays (EIA and MIA) including IL1b, IL1Ra, IL2, IL4, IL5, IL6, IL8, IL10, IL12 (p70), IL13, IL15, IL17, IL18, sCD40L, G-CSF, GM-CSF, IFNg, MCP-1, MIP-1a, MIP-1b, TGFa, TNFa, VEGF, Rantes, TGF-1
- Custom culturing and processing.

- Mycobacterium tuberculosis (TB)
- Simian Betaretrovirus / Simian Retrovirus type D
- Simian T Cell Lymphotropic Virus (STLV)
- Simian Immunodeficiency Virus (SIV)
- Herpes B Virus (HVP2)
- Measles Virus (MV)
- Simian Foamy Virus (SFV)
- Rhesus Cytomegalovirus (RhCMV)
- Rhesus Rhadino Virus (RRV)
- Lymphocrypto Virus (LCV)
- Simian Virus 40 (SV40)
- Simian Varicella Virus (SVV)
- Custom/Other Tests