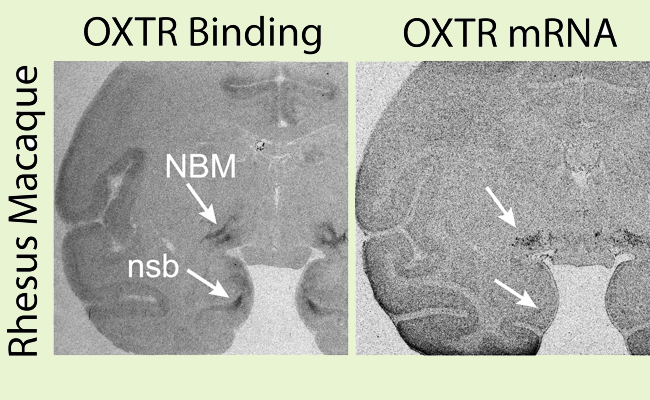
Using an exciting, first of its kind method and making great strides in understanding the biology of autism, Sara Freeman, PhD, postdoctoral researcher at the CNPRC, is the first develop a novel protocol using selective oxytocin receptor ligands to locate and map oxytocin receptors in rhesus monkey brains (“The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta)”, Freeman et al, Psychoneuroendocrinology, July:45:128-141, 2014).
Dr. Freeman has also mapped the oxytocin receptor and the closely related vasopressin 1a receptor in brain tissue from the titi monkey (“Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi money (Calicebus cupreus)”, Freeman et al, Neuroscience, July:273:12-23, 2014). Titi monkeys are small, monogamous monkeys originating in South America, and the colony at the Center is the only one of its kind in the world. Titis are involved primarily in biobehavioral studies of attachment and parental behavior and are used to understand the role of oxytocin in social behavior.
Dr. Freeman found that these two nonhuman primate species express the oxytocin receptor in areas that are important for visual, auditory, and multimodal sensory processing. This distribution is distinct from rodent brain tissue, where oxytocin receptors are concentrated in areas that process olfactory information. The hormone oxytocin is well known by scientists to play an important role in social behavior: promoting trust and cooperation and making people more attuned to social cues. This research provides insights into the neural mechanisms by which oxytocin modulates social cognition and behavior in primates, which, like humans, uses vision and audition as the primary modalities for social communication.
The information gained from the titi monkey studies has led to a fascinating investigation in which Dr. Freeman is planning to use the same novel techniques to identify receptor locations in brain tissue from normal and autistic humans. She is also using titi monkeys to evaluate an innovative protocol to noninvasively map the oxytocin receptors in living animals using PET scanning technology. These techniques will for the first time inform us where the human brain has these important receptors and will validate and vastly increase our understanding of primate models for treatment and causes of autism.
Dr. Karen J. Parker, Stanford University Medical School professor of psychology and CNPRC Affiliate Scientist conducting research in the translational social neuroscience of autism, emphasizes the importance of this research “Development of state-of-the-art imaging tools to locate oxytocin receptors in the living monkey brain has extraordinary translational potential to help identify the patients most likely to benefit from oxytocin treatment.”
With the prevalence of autism currently at 1 in 68 births, Autism Spectrum Disorder is now the fastest growing developmental disability in the US, with over $60B in annual costs. In 10 years, the projected annual cost will be $200-400 billion. Yet, with early diagnosis and intervention, the cost of lifelong care can be reduced by 60% with early diagnosis.
The rhesus macaque and titi monkey are important primate models for social behavior, and recent studies have begun to explore the impact of oxytocin on social cognition and behavior. Similar to humans, macaques and titi monkeys naturally show a spectrum of sociality and have great potential for elucidating the neural mechanisms by which oxytocin modulates social cognition, which has implications for oxytocin-based pharmacotherapies for psychiatric disorders such as autism and schizophrenia.

Oxytocin receptor binding and mRNA in the rhesus macaque and titi monkey brain, showing a strong signal in the nucleus basalis of Meynert (NBM), dentate gyrus (DG) and CA1 field (CA1) of the hippocampus, and presubiculum (PSB). nsb = nonspecific binding to the closely related vasopressin 1a receptor in the rhesus macaque.